TL;DR
Doloromics focuses on understanding the signals communicated between tissues and nociceptors and developing ways to block these signals to restore homeostasis and eliminate chronic pain.
Latch provided an accessible, customizable platform that streamlined their data analysis and target validation process, enabling them to triple the number of therapeutic targets in their pipeline.
300% increase in validated drug targets
Biologists are now able to easily access, ask questions, run custom analyses, and query data, leading to 3x faster target drug target validation.
Reduced informatics onboarding time from days to “instant”
Everyone from intro-level scientists to skilled bioinformaticians are able to simply log on and access the team’s data infra, whereas they previously had to learn AWS.
Enabled custom data analysis at scale
The Latch platform allowed Doloromics to deploy internal single-cell pipelines, notebooks, and code at scale.
Doloromics’ Story
Doloromics is an end-to-end human-to-human discovery and validation platform for identifying novel targets for the treatment of chronic pain. They focus on understanding the signals communicated between tissues and nociceptors and developing ways to block these signals to restore homeostasis and eliminate chronic pain.
They collaborate with organ donor organizations to access human sensory tissue, as well as tissues from the human periphery that are innervated by specialized sensory neurons called nociceptors.
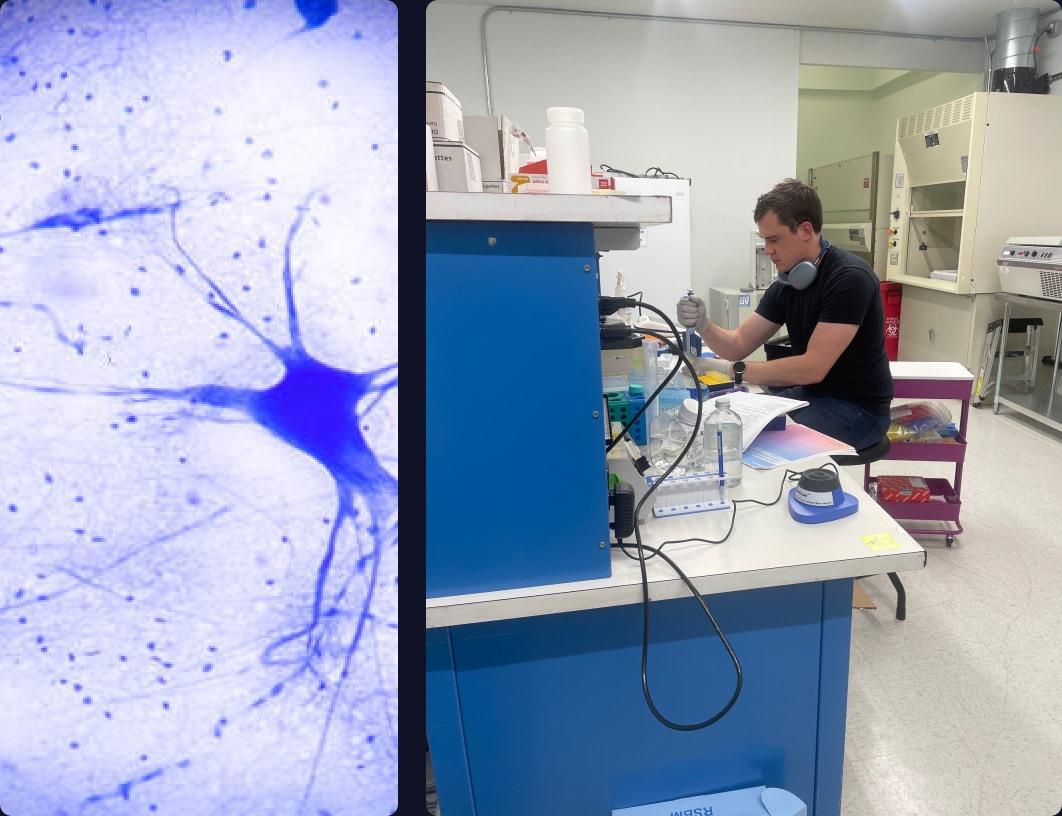
Nociceptors allow you to sense the world around you. In an individual with chronic pain, these are what transmits their chronic pain signal.
We know that nociceptors interact with tissues - they innervate - in different ways. What we can actually do is look at the signals communicated between the tissues of innervation. And identify how those signals and mechanisms are changing in different types of chronic pain. Our aim is to develop disease-modifying therapeutics to eliminate chronic pain.”
An “Atlas of Pain”
Prior to joining the Illumina Accelerator, the team at Doloromics banked 100s of tissue samples of Dorsal Root Ganglia (DRG). They leveraged Illumina’s unlimited access to sequencing for Bulk and Single-cell RNA, sequencing all their tissue. This created the most comprehensive atlas of the human DRG.
[The sequencing data is] not just from healthy individuals, but individuals with varying different forms of neuropathic pain.
The tissue is difficult to work with due to a number of factors, such as cell morphology. So my responsibility was establishing the scientific workflows and analyzing the sequencing runs from various different sequencing techniques we were piloting.
From there, we started to build out that Atlas itself. Now, we’ve taken that data and we have used it to inform the generation of a stem cell line that we differentiate into sensory neurons that allows us to validate the therapeutic targets that we found.
So we essentially go from human tissue and identifying targets from patients with chronic pain, to recapitulating the disease microenvironment within a cell culture, to be able to then test therapeutics that we’re developing and validate therapeutic targets.
As you can imagine, this requires a significant bit of single-cell sequencing to plot differentiation of cells at different time points. We need to ensure the environment we are testing in accurately recapitulates the human tissue.”
The Need for Data Infrastructure
Leaving the Illumina Accelerator, the team had built the largest and most comprehensive atlas of sequencing data on the human DRG. The challenge was now to analyze the data.
Here we are with tons and tons and tons of data... From the beginning, I wanted data to be accessible to everyone in the organization. I played around with Galaxy, s3 buckets, and many options. We considered on-site servers.

I knew this was unsustainable. As a co-founder of a startup, we have choices to make as it pertains to hiring - do we hire an incredibly talented bioinformatician or an incredibly talented scientist in pain research? And what if we could combine those?
Whoever we hired, I wanted everyone to access the data. I really wanted to level that playing field. Coming from neuroscience, I didn’t understand why there is such a walled garden around bioinformatics.
Increasing access to that walled garden by making tools just a bit more accessible - this is what drew me to Latch. I wanted us to be able to grow: to hire scientists, bioinformaticians, anybody, without them worrying about a skillset deficit.”
The Adoption of Latch
The team went with the pain scientist and started looking for data & analysis tooling. They had played around with existing tools, but were really focused on accessibility to their team.
Onboarding our lead scientist onto Latch and getting her acquainted with our data was painless. It allowed her more time to get familiar with the data we had generated. This was huge. And this really leveled that playing field.
Now, if I hire a bioinformatician tomorrow, Latch enables them to work with their own workflows in a familiar, custom environment. And if I went and hired 5 wet lab scientists tomorrow, they could also work in Latch. It’s accessible for both.”

Accelerating Drug Target Discovery
With a platform in place to easily access, explore, and interpret data together, doloromics started enabling various scientists to mine their datasets for new targets, without the need for extensive bioinformatics training.
The increased accessibility to data has led us to generate new therapeutic targets. It’s increased our ability to quickly query data to find out if we have expression of different markers, to identify new biomarkers for different types of pain, and to validate those targets.
Last July, we had 4-5 validated targets in our pipeline. In the year since then, we’ve increased that to 12-15 targets. The number of targets in our pipeline has tripled.
We have this entire menu of therapeutic targets we have validated now. And we are able to rapidly generate these in pain, and other areas of science.”
Doloromics continues to push the edges of pain science. And we feel honored to play a small role in the advancement of their science. To learn more, go to doloromics.com.
The increased accessibility to data has led us to generate new therapeutic targets. It’s increased our ability to quickly query data to quantify gene expression in targeted tissues, to identify new biomarkers for different types of pain, and to validate those targets”
Jackson Brougher, Chief Scientific Officer, Doloromics


